Oil refinery involves the process of fractional distillation of crude oil to produce lubricating oils that meet the specific requirements of engines. The engine oil refinery process involves heating the crude oil and separating it into different fractions based on their boiling points. The resulting fractions are then further refined to produce high-quality engine oils that can withstand high temperatures and pressures while providing excellent lubrication and protection to engine components.
Fractional distillation is the backbone of modern society, providing a wide range of essential products, including fuels, lubricants, and chemicals. In this article, we will explore the principles of fractional distillation, the various products obtained from the process, and their applications.
We will also discuss the factors influencing the distillation process and the challenges and future of the industry. By understanding the complex process of fractional distillation, we can better appreciate the role it plays in our daily lives and the importance of developing sustainable solutions for the future.
Table of Contents
The Process of Fractional Distillation of Crude Oil
In this section, we will discuss the process of fractional distillation, including the design and working principle of distillation columns, the separation of hydrocarbons based on boiling points, and the key factors that affect the distillation process.
Pre-Process Treatment of Crude Oil
Before crude oil can be fractionally distilled, it must first undergo pre-treatment, which involves removing impurities such as water, sulfur, and other contaminants. This is done to ensure that the crude oil does not damage the distillation column or affect the quality of the end products.
Overview of the Fractional Distillation Process
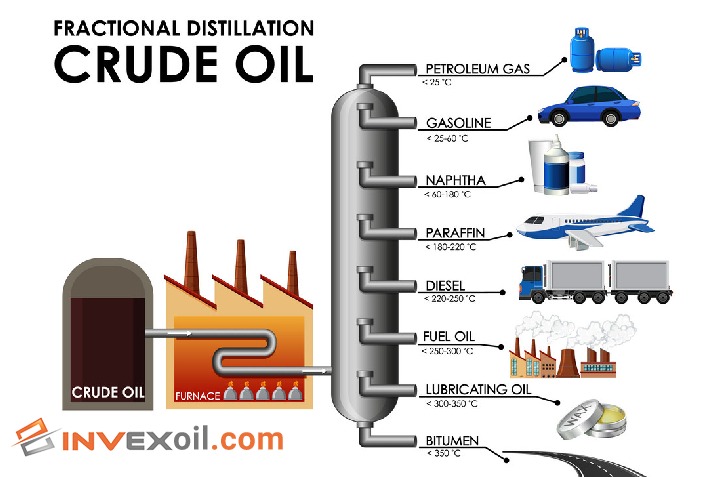
The fractional distillation process involves heating the crude oil to a high temperature and passing it through a distillation column. The column is designed to separate different hydrocarbon compounds based on their boiling points, with the lighter fractions rising to the top of the column and the heavier fractions settling at the bottom.
Distillation Column Design and Working Principle
Distillation columns are typically made up of several trays or plates, which provide a large surface area for the vaporization and condensation of the different fractions. The working principle of the distillation column is based on the difference in boiling points of the various hydrocarbon compounds in crude oil.
Separation of Hydrocarbons Based on Boiling Points
The separation of hydrocarbons in the distillation column is achieved by controlling the temperature at different levels of the column. The lighter hydrocarbons with lower boiling points will vaporize at lower temperatures, while the heavier hydrocarbons with higher boiling points will condense at higher temperatures.
Key Factors Affecting fractional distillation of crude oil Process
Several factors can affect the fractional distillation process, including the temperature and pressure in the distillation column, the flow rate of crude oil, and the composition of the crude oil itself. These factors can impact the efficiency of the process and the quality of the end products.
Distillation Efficiency and Product Quality
The efficiency of the fractional distillation process is determined by the number of trays in the distillation column and the operating conditions. The quality of the end products is also affected by the efficiency of the process, as well as the composition of the crude oil and the refining process used.
Types of Distillation Columns and Their Specifications
| Type of Column | Specifications |
| Simple | No internal packing material, small diameter |
| Fractionating | Multiple trays or plates, packing material, larger diameter |
| Packed | Large surface area, efficient for separating high-boiling point compounds |
| Steam | Uses steam to vaporize the crude oil, efficient for separating low-boiling point compounds |
Products Obtained from Fractional Distillation of Crude Oil
The products obtained from this process include gases, gasoline, kerosene, diesel, fuel oil, and residue. Gases such as methane, ethane, propane, and butane have low boiling points and are typically used as fuel or feedstock for other processes.
Gasoline, which is used as fuel for vehicles, is obtained from the fraction with boiling points ranging from 30-200 °C. Kerosene, with a boiling range of 150-300 °C, is used as fuel for jet engines and heating oil. Diesel, with a boiling range of 250-350 °C, is used as fuel for diesel engines. Fuel oil, which has a boiling range of 350-600 °C, is used for heating and generating electricity. Residue, which has the highest boiling point, is used as asphalt or lubricating oil.
Chemical Composition of Products
The composition of the various distillates obtained from crude oil depends on the hydrocarbons present in the original crude oil. The composition of the products determines their physical properties, such as boiling point, viscosity, and density, which in turn affects their applications.
For example, gasoline has a higher octane rating due to the presence of aromatic hydrocarbons, while diesel has a higher cetane rating due to the presence of alkanes. The chemical composition of the products obtained from fractional distillation is complex, with a mixture of straight-chain, branched-chain, and cyclic hydrocarbons.
Table: Properties of various distillates
| Distillate | Boiling Range (°C) | Density (g/cm3) | Viscosity (cSt) | Application/Use |
| Gas | Below 25°C | 0.7 – 0.8 | Low | Fuel for automobiles and cooking |
| Naphtha | 25-200°C | 0.7 – 0.8 | Low | Used in gasoline blending and the petrochemical industry |
| Kerosene | 150-300°C | 0.78 – 0.81 | Moderate | Jet fuel, heating fuel |
| Diesel | 200-350°C | 0.83 – 0.86 | Moderate | Fuel for diesel engines and generators |
| Residual Fuel Oil | Above 350°C | 0.95 – 1.03 | High | Power generation and heating |
Factors Influencing Fractional Distillation of Crude Oil
several factors impact the efficiency and yield of the process. Some of these factors include:
Composition of Crude Oil
The chemical composition of crude oil, which varies depending on the source, influences the boiling points of the hydrocarbons and thus, the separation efficiency of the process.
Boiling Points of Hydrocarbons
The boiling points of the various hydrocarbons present in crude oil also play a crucial role in determining the efficiency of the fractional distillation process.
Heat Transfer Rates
The rate of heat transfer within the distillation column affects the efficiency of the separation process. High heat transfer rates lead to more efficient separation of hydrocarbons.
Distillation Column Design and Operation
The design and operation of the distillation column, including the number of trays or packing, the temperature and pressure conditions, and the reflux ratio, also affect the efficiency of the separation process.
Refinery Operating Conditions
The operating conditions in the refinery, such as temperature and pressure, can also influence the efficiency and yield of the fractional distillation process.
Applications of Fractional Distillation
Fractional distillation has different applications including:
Petroleum Refining
The fractional distillation of crude oil is an essential process in petroleum refining. It separates crude oil into its various components, which can be further processed into gasoline, diesel, kerosene, and other products.
Chemical Manufacturing
Fractional distillation products such as naphtha, propylene, butadiene, and benzene are used in the production of chemicals such as plastics, rubber, solvents, and detergents.
Fuel Production
Fractional distillation products such as gasoline, diesel, and jet fuel are used for transportation and heating.
Petrochemicals
Fractional distillation products such as ethylene, propylene, and butadiene are used to produce petrochemicals such as polyethylene, polypropylene, and synthetic rubber.
Other Applications
Fractional distillation is also used in the production of lubricating oils, asphalt, and other products.
Challenges and Future
the fractional distillation of crude oil faces its share of challenges, particularly in the areas of environmental impact, feedstock quality, and competition from alternative energy sources. The refining process can generate greenhouse gas emissions and other pollutants that can have harmful effects on the environment. In addition, the quality of crude oil feedstock can vary widely, making it difficult to achieve consistent product quality and refining efficiency.
The development of alternative energy sources, such as solar, wind, and biofuels, has also impacted the demand for traditional petroleum-based products. However, while the use of alternative energy sources continues to grow, crude oil and its derivatives are expected to remain a major source of fuel and feedstock for the foreseeable future.
To address these challenges, the industry is exploring new technologies and processes to reduce environmental impact and improve efficiency. These include carbon capture and storage, the use of renewable energy sources in the refining process, and improvements in feedstock quality control.
Conclusion
fractional distillation of crude oil is a critical process that is used to separate crude oil into its various components, which are then used for a range of applications, including fuel production, chemical manufacturing, and petrochemicals.
The process involves the careful design and operation of distillation columns, which separate hydrocarbons based on their boiling points. While the process has faced challenges, including environmental concerns and the need for alternative energy sources, fractional distillation remains a key process in the refining and processing of crude oil for the future.
FAQ
What is a fractional distillation of crude oil?
Fractional distillation is a process of separating crude oil into different components based on their boiling points.
What are the products obtained from fractional distillation of crude oil?
The products include gasoline, diesel, kerosene, and other distillates.
What are the key factors affecting the efficiency of fractional distillation of crude oil?
The key factors affecting the efficiency of fractional distillation of crude oil include the composition of crude oil, boiling points of hydrocarbons, heat transfer rates, distillation column design and operation, and refinery operating conditions.







In Onliner.co, Our team with 12 professional members in each of content creation, on-page, off-page, and technical domain has always been able to achieve customer satisfaction with 12 years of experience in SEO. In the content creation sector, our expert writers are up to date and experienced to provide a high quality and unique contents.